Hf Lewis Structure Shape
Things to remember 1.
Hf lewis structure shape. 3 l p 1 sigma bond sp3 tetragonal geometry like paraffin ch4. Carbon c will always be the central atom and hydrogen h will never be the central atom 2. For all practical purposes they are considered the same chemical.
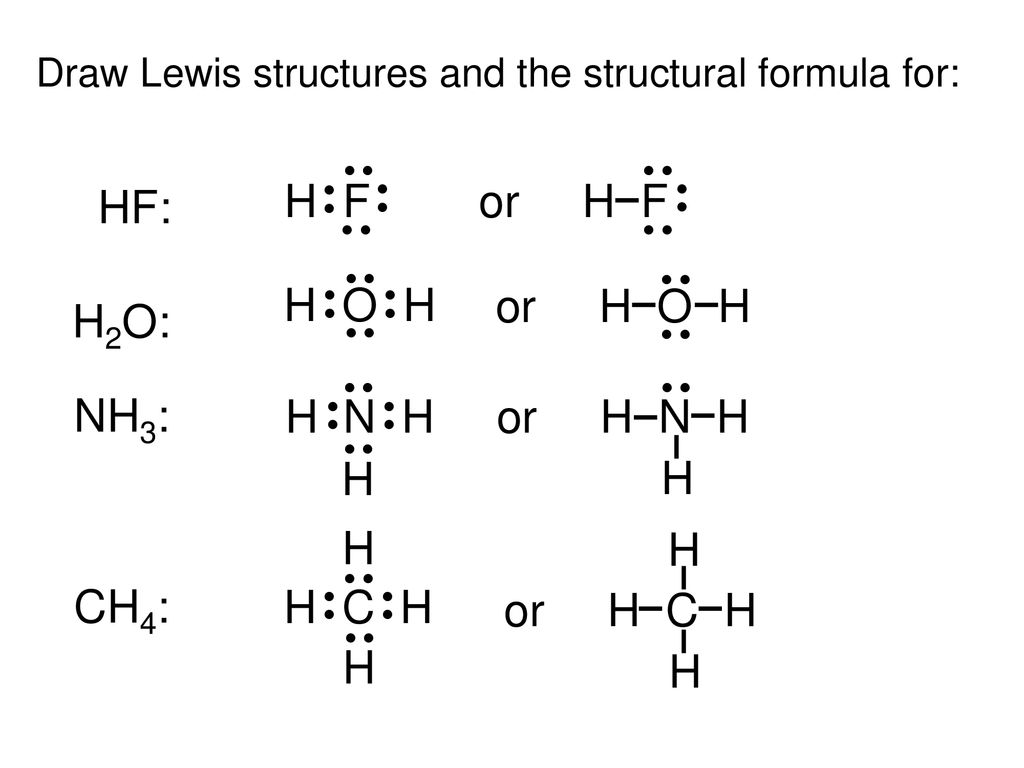
However a question may arise that the bonds with the formation of charges are considered ionic in nature. To know hybridisation count no. Drawing the lewis structure for hf.
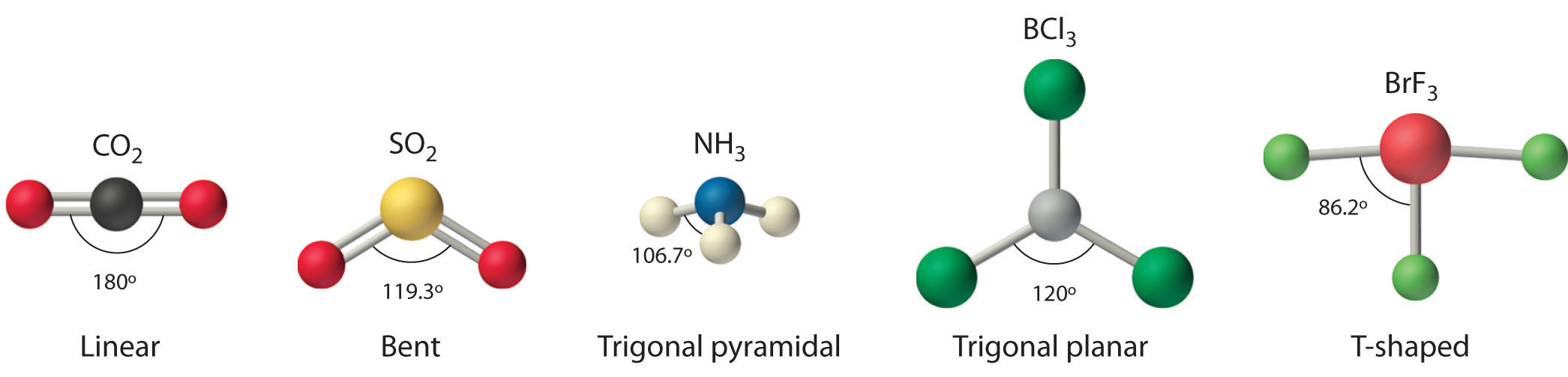
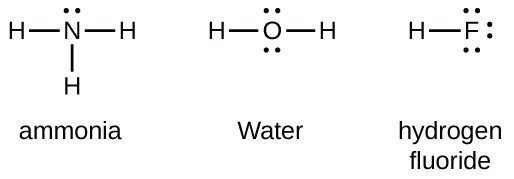
Below is the geometrical shape of the lewis structure of hf. Cl 2 r. Lewis structures are not generally expected to represent the geometrical shape of a molecule one reason being that they must be drawn in a plane while the molecule itself may not be planar.
It is an intermediate for many chemical reactions and syntheses. Of lone pairs and no. N 2 o i.
Hydrogen fluoride mixes readily with water forming hydrofluoric acid. A lewis structure is expected to show a which atoms in the molecule are bonded together and. Hf is very similar to hf and hcl.
In other cases choose the element that is the least electronegative farthest to the left on the periodic table. Florine has 7 electrons in its valence shell. H 2 o m.
Hydrogen has 1 valence electron and fluorine in group 7 with f and cl has 7 valence electrons. Lewis structures shapes and polarity w 319 everett community college student support services program draw lewis structures name shapes and indicate polar or non polar for the following molecules. Ch 2 o f.
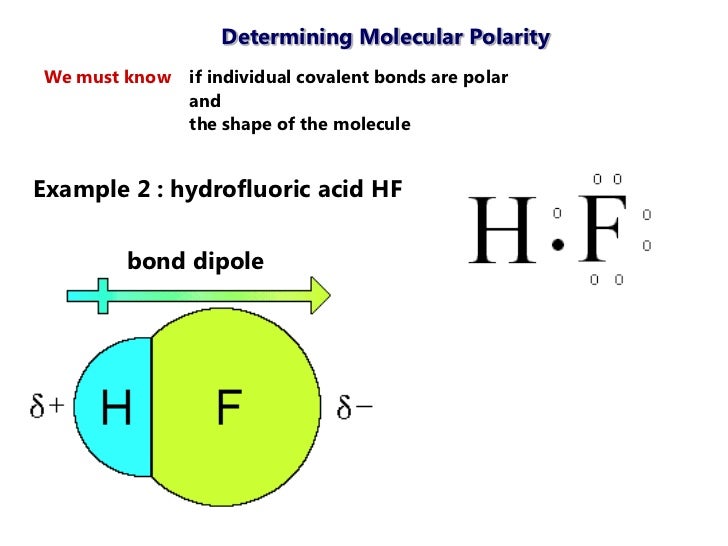
Polar vs ionic bond. Draw the lewis structure then id shape and polarity. These positive and negative charges lead to the formation of a net dipole on the hf molecule.
Gaining one extra electron would result as octet completion. Drawing a lewis structure is the first steps towards predicting the three dimensional shape of a molecule. Ccl 2 f 2 d.
Hydrogen fluoride hydrofluoric acid is used extensively in the extraction processing and refining of metals rock brick and oil. No need to count no. To do so 3 lone pairs would left vacant.